Navigating the Future of MedTech Regulation: Reflections on the MHRA Reform

The Medicines and Healthcare products Regulatory Agency (MHRA)’s roadmap, unveiled on 9 January 2024, marks a significant stride towards the future of medical device regulation in the UK.
The MHRA’s reform on MedTech regulatory framework aims to position the UK as a frontrunner in medical device safety and innovation. Emphasising patient safety and aligning with international best practices, this reform seeks to introduce a more flexible, responsive, and proportionate regulatory environment for medical devices, including software and AI-driven technologies.
Key Highlights:
Enhanced Patient Safety: The reform prioritises patient safety, aiming for stringent post-market surveillance and incident reporting.
International Harmonisation: Aiming for closer alignment with global best practices, facilitating easier market access for innovative medical technologies.
Inclusive Regulation: Expanding the definition of medical devices to include modern technological advancements like AI and software, ensuring they meet safety standards.
Stakeholder Engagement: Continual consultation with industry stakeholders to ensure realistic and proportionate regulatory measures.
Gradual Implementation: Introduction of changes through Statutory Instruments, prioritising aspects most beneficial to patient safety.
The MHRA MedTech Regulatory Reform
This reform, set against the backdrop of technological advancements and post-Brexit transitions, presents a pivotal shift in how medical devices are regulated in the UK. This transformation, detailed in a comprehensive webinar, outlines the agency’s strategic vision to uphold public health by fostering a regulatory ecosystem that is at once rigorous and adaptable to innovation.
At its core, the reform is designed to enhance patient and public safety by tightening the controls around medical devices, including in vitro diagnostic devices (IVDs) and software as medical devices (SaMD).
Recognising the dynamic nature of medical technology, particularly with the rise of artificial intelligence (AI), the MHRA is keen on establishing a framework that not only addresses current needs but is also forward-looking.
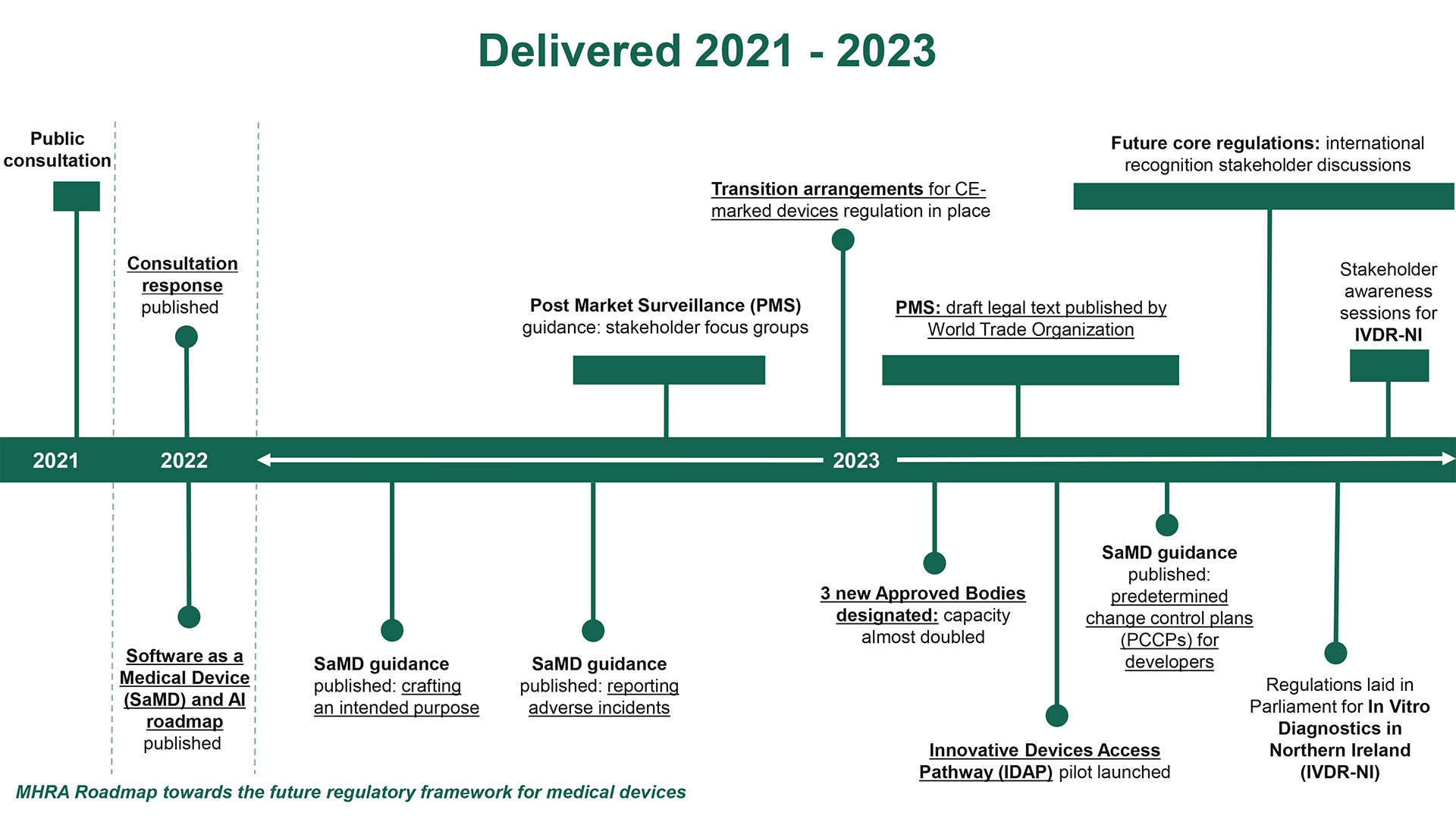
The 2021 public consultation underscored the healthcare community’s call for an overhaul. Stakeholders expressed a strong desire for improved patient safety measures and streamlined access to high-quality, effective medical devices. This feedback has been instrumental in shaping policies that strengthen post-market surveillance, enhance device traceability, and embrace international recognition routes to reduce industry burdens.
The MHRA’s methodical approach to reform involves the phased introduction of new regulations, commencing with extended CE mark acceptance to mitigate supply risks linked to Brexit. Ambitious projects like the software and AI as medical device change program illustrate the agency’s commitment to modernizing its regulatory approach, ensuring it keeps pace with technological advancements.
Innovation is at the heart of the reform, with initiatives like the Innovative Devices Access Pathway (IDA) aiming to expedite patient access to cutting-edge medical technologies. The IDA pilot scheme, a collaborative effort with health authorities, has already identified promising products for expedited review, signaling the MHRA’s dedication to fostering innovation while safeguarding patient health.
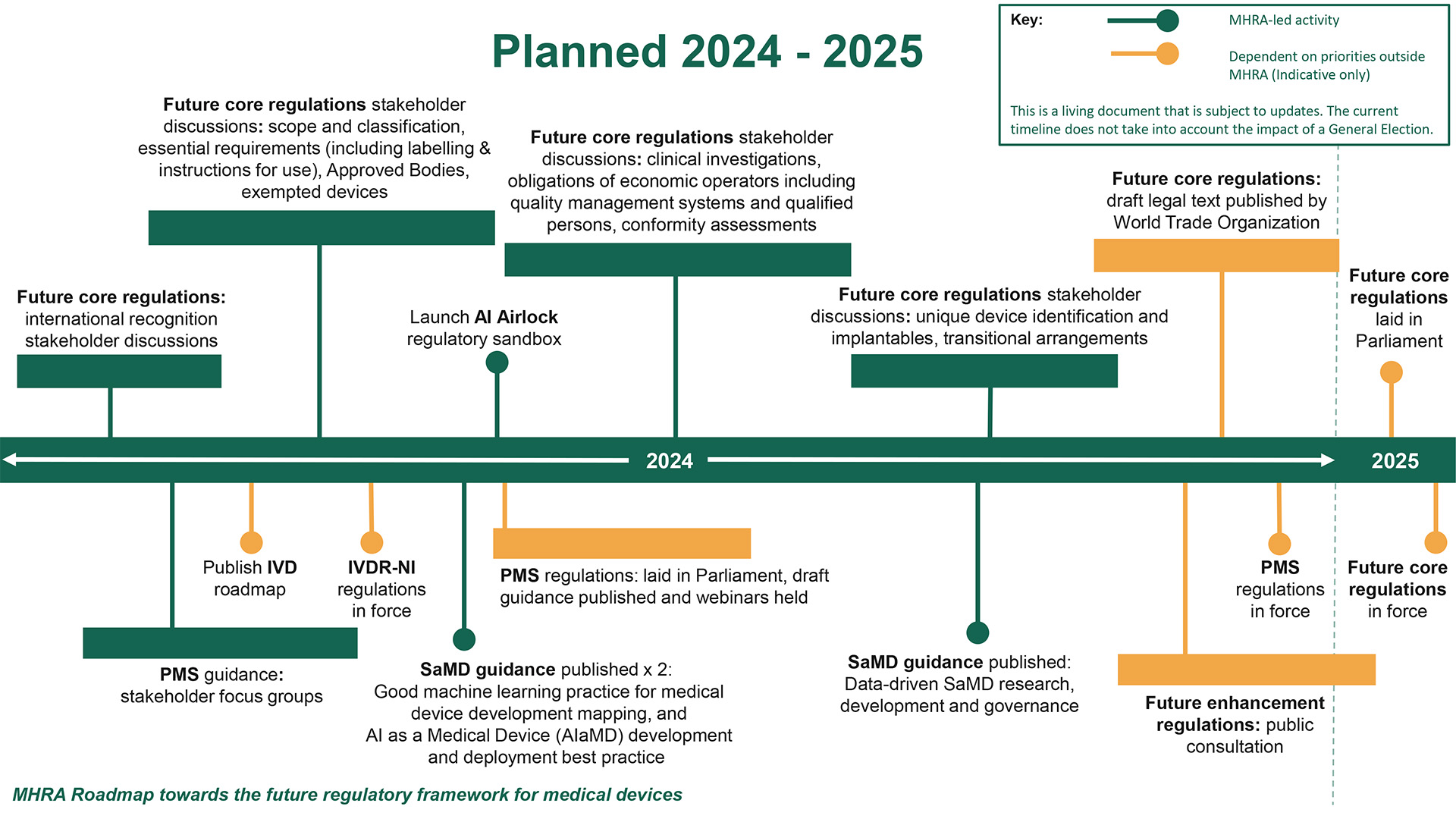
The regulatory roadmap released by the MHRA provides a clear timeline for the introduction of new laws, emphasizing stakeholder engagement throughout the process. This transparent and inclusive approach is designed to ensure that the forthcoming regulations are not only effective but also equitably applied across the medical device spectrum.
To address the diverse and evolving landscape of medical devices, the reform includes a re-evaluation of device classifications. This adjustment ensures that devices are appropriately categorized based on risk, facilitating a more targeted and effective regulatory oversight. For software and AI-driven devices, the MHRA proposes a definition that captures the essence of these technologies while paving the way for specialized regulatory pathways.
In conclusion, the MHRA MedTech Regulatory Reform represents a significant leap towards establishing a regulatory framework that balances innovation with patient safety. By adopting a patient-centered approach and aligning with global standards, the MHRA is setting the stage for the UK to lead in the safe and effective use of medical technologies. As the reform unfolds, continued dialogue with stakeholders will be vital in refining and implementing these regulatory enhancements, ensuring that the UK remains at the forefront of healthcare innovation.
Let’s Chat! Book Your Free Strategy Session Now
Peyman Moh
Peyman Moh, a seasoned leader with over 20 years of experience in digital health and innovation, excels in transforming foresight into impactful realities. As the former Director of Digital Health & Innovation at GSK and founder of Foretell Innovation Lab, he has spearheaded major projects, established innovation accelerators, and provided advisory services. Renowned for his strategic foresight and ability to foster ecosystem collaborations, Peyman's expertise in future-back thinking and innovation lifecycle management positions him as a pivotal figure in navigating the rapidly evolving innovation landscape.